austin5053
15.11.2020 •
Chemistry
The graph above shows the distribution of molecular speeds for four different gases at the same temperature. What property of the different gases can be correctly ranked using information from the graph, and why?
(Graph attached) I WILL MARK BRAINLIEST
A.) The densities of the gases, because as the density of a gas increases, the average speed of its molecules decreases.
B.) The pressures of the gases, because the pressure exerted by a gas depends on the average speed with which its molecules are moving.
C.) The volumes of the gases, because at a fixed temperature the volume of a gas can be calculated using the equation PV=nRT.
D.) The molecular masses of the gases, because the gas molecules have the same average kinetic energy and mass can be calculated using the equation KEavg=12mv2
Solved
Show answers
More tips
- F Family and Home How to Properly Use a Water Level?...
- D Dating, Love, Relationships 10 Useful Tips on How to Survive a Breakup?...
- F Food and Cooking Apple Cider Vinegar: The Ultimate Health and Beauty Solution...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
Answers on questions: Chemistry
- C Chemistry 1. how many valence electrons are in an atom of phosphorus? (atomic number 15) a. 2 b. 3 c. 4 d. 5 2. how many electrons does barium have to give up to achive a noble-gas electron...
- C Chemistry Which of these would you expect to be soluble in the nonpolar solvent carbon disulfide, cs2?...
- C Chemistry Molly just started a new job. She needs to fill out a so that her employer knows how much tax to take out. 1099 form tax return form W-4 form W-2 form...
- C Chemistry The table below compares the radioactive decay rates of two materials. Material Original mass of material (in grams) Mass of material after 60 hours (in grams) 1 208 13 2 200...
- C Chemistry 5. The partition coefficient of Compound A is 7.5 in dichloromethane (a.k.a. methylene chloride) with respect to water. a. If 5 grams of Compound A were dissolved in 100 mL...
- C Chemistry Which reactions have a positive Δrxn? A(g)+B(g)⟶C(g) 2A(g)+2B(g)⟶5C(g) A(s)+B(s)⟶C(g) 2A(g)+2B(g)⟶3C(g)...
- C Chemistry Consider the reaction 2AI(OH)3 + 3H2SO4 Right arrow. X + 6Y. What are X and Y? X = AI2(SO4)3; Y = H2O X = AI2(SO4)3; Y = H2 X = AI2(SO3)3; Y = H2O X = AI2(SO3)3; Y = H2...
- C Chemistry A gas-forming reaction produces 1.45 m3 of gas against a constant pressure of 112.0 kPa. Calculate the work done by the gas in joules....
- C Chemistry The large molecule represented in figure 2 is an atom a metal a polymer a salt...
- E English which of the following is not an important feature of technical directions? a. numbered steps to follow b. a long historical account of how the device is used c. a diagram of...

Ответ:
The correct option is;
D.) The molecular masses of the gases, because the gas molecules have the same average kinetic energy and mass can be calculated using the equation,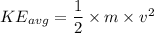
Explanation:
The graph shows the proportion of the atoms of each gas have a given velocity
The given parameters of the graphs are;
The dependent variable of the graph = The number of molecules
The independent variable = The molecular speed (m/s)
The temperature of the gases = The same temperature
Therefore, from the above equation, at constant temperature, the root mean square velocity varies inversely as the molecular weight
Similarly from the kinetic energy equation, we have;
Whereby, the energy contained in each of the four gas are the same, we have;
For increasing molecular mass by a factor of 2, the velocity decreases by a factor of 4.
Ответ:
social entrepreneurship aimed to obtain as much profit as possible while providing aids for both social and environmental issues at the same time.
Often times, both of these aims contradict each other, which lead to the Ethical Dilemma
For example, in order to obtain maximum profit, it is far cheaper to dump production waste to the river rather than spending money to create proper waste management system.