mustachegirl311
10.09.2019 •
Chemistry
The reaction described by the equation ch 3 cl + naoh → ch 3 oh + nacl follows the second-order rate law, rate = k [ ch 3 cl ] [ naoh ] . when this reaction is carried out with starting concentrations [ ch 3 cl ] = 0.2 m and [ naoh ] = 1.0 m , the measured rate is 1 × 10 − 4 mol l − 1 s − 1 . what is the rate after one-half of the ch 3 cl has been consumed? (caution: the initial concentrations of the starting materials are not identical in this experiment. hint: determine how much of the naoh has been consumed at this point and what its new concentration is, compared with its initial concentration.)
Solved
Show answers
More tips
- H Health and Medicine What is Autism? Understanding the Basics of This Neurodevelopmental Disorder...
- F Family and Home How to Properly Use a Water Level?...
- D Dating, Love, Relationships 10 Useful Tips on How to Survive a Breakup?...
- F Food and Cooking Apple Cider Vinegar: The Ultimate Health and Beauty Solution...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
Answers on questions: Chemistry
- C Chemistry Which of the following provides the best description of the function of genes? A. Ridding individual cells of waste products. B. Relaying information from the environment....
- C Chemistry How the lead (II) carbonate can be separated and collected from the product mixture with ammonium nitrate?...
- C Chemistry 30g of solute is dissolved in 100g of water. Calculate its % (m/m) concentration....
- C Chemistry Two water molecules are separated by 2.76 Å in air. Use equation 13.9 to calculate the dipole-dipole interaction (in kJ/mol). The dipole moment of water is 1.82 D. Hint:...
- C Chemistry Look at the H-R diagram. Which area of the diagram represents the white dwarfs? Give answer like top, right corner....
- C Chemistry Question 2 (1 point) An object s gravitational force depends primarily on the object s a density mass b oc momentum...
- B Business a calculation that summarizes the expected costs, revenues, or profits of a capacity alternative based on several demand levels, each of which has a different probability...
- S Social Studies Researchers put a man in a hot pink shirt and had him walk through the mall food court. afterwards, they asked the man how many people noticed he was wearing a hot pink...
- P Physics Acar of mass 1230 kg is on an icy driveway inclined at an angle of 39°. the acceleration of gravity is 9.8 m/s². θ if the incline is friction-less, what is the acceleration...
- B Business Smart company rarely had to write down inventory. in the past, when inventory write-downs were necessary, the company debited cost of goods sold. recently, write-downs...

Ответ:
The rate is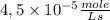
Explanation:
Stoichiometry
Kinetics
The rate constant K can be calculated by replacing with the initial data
Taking as a base of calculus 1L, when half of the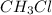 is consumed the mixture is composed by
is consumed the mixture is composed by
Then, the rate is
The reaction rate decreases because there’s a smaller concentration of reactives.
Ответ:
Absorbed
Released
Released
Explanation:
The formation of a cation is an endothermic process because energy must be absorbed in order to remove an electron from an atom.
Similarly, energy is evolved when an electron is added to an atom to form a negative ion.
The formation of an ionic compound is an exothermic process. Since ionic compounds are more stable than the individual ions separated by a distance, the excess energy of the isolated ions is evolved when the ionic compound is formed.