xxgissellexx
03.12.2019 •
Chemistry
The reaction described by this equation
o3(g)+no(> o2(g)+no2(g)
has the following rate law at 310k.
rate of reaction=k[o3][no] k=3.0*10^6m^-1*s^-1
given that [o3]=5.0x10^-4m and no=6.0x10^-5m at t=0 calculate the rate of the reaction at t=0
what is the overall order of this reaction?
Solved
Show answers
More tips
- F Food and Cooking How to Make Thin Pancakes: Recipe and Tips...
- S Style and Beauty Is Hot Scissor Haircutting Beneficial or Dangerous?...
- S Style and Beauty How to Get Rid of Under Eye Bruises?...
- F Food and Cooking Is Bacon Good for You?...
- S Style and Beauty Discover the Art of Nail Design: How Do You Paint Your Nails?...
- P Philosophy How to Develop Extrasensory Abilities?...
- O Other Everything You Need to Know About Kudyabliks...
- C Computers and Internet The Twitter Phenomenon: What it is and How to Use it...
- C Computers and Internet How to Choose a Laptop: Expert Guide and Tips...
- C Computers and Internet How to Choose a Monitor?...
Answers on questions: Chemistry
- C Chemistry Which compound reacts with an acid to produce water and a salt (1) ch3cl (2) ch3cooh (3)kcl (3)koh...
- C Chemistry What s the purpose of mitosis and meiosis?...
- C Chemistry List and describe, in your laboratory journal, the four forces acting on amino acids placed in water. these four forces ultimately determine the shape of a protein. write definitions,...
- C Chemistry What is the velocity of a BB pellet that is 0.02kg and has 36 J of KE...
- C Chemistry When aqueous solutions of ammonium carbonate and zinc iodide are combined, solid zinc carbonate and a solution of ammonium iodide are formed. The net ionic equation for this...
- M Mathematics 7+3(s-4t) simplify the expression...
- S Social Studies Can some one please help me.???...
- B Biology Worth 30 as i don’t understand! match each image shown in the picture with a phase in the cell cycle. interphase prophase metaphase anaphase telophase cytokinesis ex: image 1...
- E English All this comes about particularly because i have in my mind s eye an image of what a perfect mother and wife should be; and in her whom i must call mother i find no trace of...
- M Mathematics What is the sum of the infinite geometric series?...

Ответ:
Answer :
The rate of the reaction at t=0 is,
The overall order of reaction is, second order reaction.
Explanation :
Rate law : It is defined as the expression which expresses the rate of the reaction in terms of molar concentration of the reactants with each term raised to the power their stoichiometric coefficient of that reactant in the balanced chemical equation.
The general reaction is:
The general rate law expression for the reaction is:
where,
a = order with respect to A
b = order with respect to B
R = rate law
k = rate constant
Now we have to determine the rate law for the given reaction.
The given balanced equations is:
In this reaction,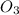 and
and  are the reactants.
are the reactants.
The rate law expression for the reaction is:
Given :
Rate constant =
Concentration of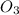 =
= 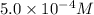
Concentration of =
= 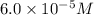
Now put all the given values in equation 1, we get:
Thus, the rate of the reaction at t=0 is,
Now we have to determine the overall order of reaction.
From the given rate law expression we conclude that,
The order of reaction with respect to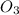 is, first order reaction.
is, first order reaction.
The order of reaction with respect to is, first order reaction.
is, first order reaction.
Thus, overall order of reaction will be:
Overall order of reaction = 1 + 1 = 2
Thus, the overall order of reaction is second order reaction.
Ответ:
increase
Explanation: