katheline1226
18.01.2021 •
Chemistry
The reaction of hydrogen sulfide(g) with oxygen(g) to form water(l) and sulfur dioxide(g) proceeds as follows: 2H2S(g) 3O2(g)2H2O(l) 2SO2(g) When 9.82 g H2S(g) reacts with sufficient O2(g), 161 kJ is evolved. Calculate the value of rH for the chemical equation given. kJ/mol
Solved
Show answers
More tips
- F Food and Cooking What s the Best Rice for Cooking Plov?...
- F Family and Home How to Remove Fading from Clothes: Tips and Tricks...
- F Food and Cooking How to Make Polendwitsa at Home?...
- F Family and Home Parents or Environment: Who Has the Most Influence on a Child s Upbringing?...
- P Philosophy Unbelievable stories of encounters with otherworldly forces...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
Answers on questions: Chemistry
- H History How many countries are involved in manufacturing/trade in Africa?...
- A Arts Motivate why sophiatown will be a suitable choice for a school production...
- B Biology Female frogs lay eggs in the water.male frogs release their sperm into the water to fertilize the eggs.this is an example∵∴∵∴∵∴∵∴∵∴∵∴∵∴∵∴∵∴∵∴∵∴∵∴∵∴∵∴∴∵∴...
- M Mathematics Solve the equation y=x²-2x-24...

Ответ:
The value of is 1118 .
is 1118 .
Explanation:
To calculate the number of moles, we use the equation:
The balanced chemical reaction is:
Given :
Energy released when 0.288 mole of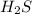 is reacted = 161 kJ
is reacted = 161 kJ
Thus Energy released when 2 moles of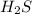 is reacted =
is reacted = 
Thus the value of is 1118.
is 1118.
Ответ: