juliannabartra
17.12.2020 •
Chemistry
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and delta H rxn given above, which of the following can best be used to justify that the reaction is thermodynamically favorable at 298 K and constant pressure?
N2 (g) + 3H2 (g) --> 2NH3 (g)
K= 5.6 × 10^5 at 298 K
delta H rxn= -91.8 kj/mol
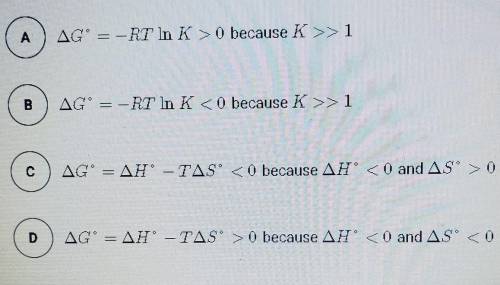
Solved
Show answers
More tips
- S Science and Technology QR Code: How it Works and Why You Need it?...
- C Computers and Internet Dropbox: What is it and How to Use it...
- H Health and Medicine How to Increase Hemoglobin in the Blood...
- A Animals and plants How to Store Carrots: Tips for Homeowners...
- L Legal consultation Juvenile Justice: Who Needs It?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
Answers on questions: Chemistry
- C Chemistry A 1230 kg car accelerates at a rate of 4m/s,how much force is involved?...
- C Chemistry Summarize chemical equations and their purpose...
- C Chemistry Si tenemos la siguiente configuración: 4d2. Determinar los 4 N.C. del último electrón....
- C Chemistry How much hcl in grams can be neutralized by 5.50 g of mg(oh)2?...
- C Chemistry Which of the following nuclei would be the most stable? a. 10 protons, 13 neutrons b. 10 protons, 14 neutrons c. 10 protons, 15 neutrons d. 10 protons, 12 neutrons...
- C Chemistry How many grams of cl are in .46 moles of cl?...
- C Chemistry In the following reaction, oxygen is the excess reactant. Sicla + O2-SiO2 + Cl2 The table shows an experimental record for the above reaction....
- C Chemistry Which of the following forces would take the longest amount of time to change the surface of the Earth? A. volcano B. earthquake C. tsunami D. seafloor spreading HELPP...
- H History If a majority of committee members support a bill, what is likely to happen to it? a. the committee will pigeonhole the bill b. the committee will report the bill favorably...
- M Mathematics Convert 7.2 x 10-3 to standard form...

Ответ:
d
Explanation:
Ответ:
i agree to that
Explanation: