scarlettp13
03.08.2019 •
Chemistry
The useful metal manganese can be extracted from the mineral rhodochrosite by a two-step in the first step, manganese(ii) carbonate and oxygen react to form manganese(iv) oxide and carbon dioxide: 2mnco3 + o2=2mno2 + 2co2in the second step, manganese(iv) oxide and aluminum react to form manganese and aluminum oxidide: 3mno2 + 4al = 3mn + 2al2o3 suppose the yield of the first step is 65.% and the yield of the second step is 80.%. calculate the mass of manganese(ii) carbonate required to make 8.0kg of manganese. be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.
Solved
Show answers
More tips
- W Work and Career Where can you learn to be a flight attendant?...
- C Cities and Countries What time does the Metro open in Moscow?...
- S Style and Beauty How to Get Rid of Under Eye Bruises?...
- F Food and Cooking How to Calculate the Gender of Your Child with Blood?...
- C Computers and Internet IMHO: What is it, why is it important, and how to use it?...
- H Health and Medicine Effective Ways to Treat Colic in Infants...
- H Health and Medicine How to Treat the Flu: A Comprehensive Guide...
- S Style and Beauty 5 Tips for Choosing the Best Ugg Boots...
- C Computers and Internet How to Create a Folder on Your iPhone?...
- C Computers and Internet iPhone SMS Delivery Report: How to Set It Up...
Answers on questions: Chemistry
- C Chemistry Of the following solutions, which has the greatest buffering capacity? of the following solutions, which has the greatest buffering capacity? 0.221 m hf and 0.105 m naf...
- C Chemistry Most scientific questions are developed from...
- C Chemistry The michaelis constant for pancreatic lipase is 5 mm. at 60 c, lipase is subject to deactivation with a half-life of 8 min. fat hydrolysis is carried out in a well-mixed...
- C Chemistry Write 4 important of measurement?...
- C Chemistry Atoms of the same element with a different number of neutrons are called when a neutral atom gains or loses electrons, it becomes charged and is called ain)!...
- C Chemistry What conversion process takes place in a voltaic cell? chemical energy is converted to electrical energy. electrical energy is converted to chemical energy. electrons...
- C Chemistry If I contain 8.3 moles of gas In a container with the volume of 33 liters and at a temperature of 85 Celsius, what is the pressure inside the container?(Ideal Gas Law)...
- C Chemistry 2K + F, - 2KF Calculate the mass of fluorine that reacts with 3.9 g of potassium....
- M Mathematics Aclub with 24 members has 4,896 event flyers to pass out. each member will be given the same number of flyers. how many flyers does each member receive?...
- E English Identify the subject: your mittens are on the table, next to your book bag and keys...

Ответ:
33 kg
Explanation:
Let's consider the two steps to obtain manganese.
Step 1: 2 MnCO₃ + O₂ = 2 MnO₂ + 2 CO₂
Step 2: 3 MnO₂ + 4 Al = 3 Mn + 2 Al₂O₃
The molar mass of Mn is 54.94 g/mol. The moles represented by 8.0 kg of Mn are:
8.0 × 10³ g × (1 mol / 54.94 g ) = 1.5 × 10² mol
In Step 2, the real yield of Mn is 1.5 × 10² mol and the percent yield is 80%. The theoretical yield is:
1.5 × 10² mol (Real) × (100 mol (Theoretical) / 80 mol (Real)) = 1.9 × 10² mol
According to Step 2, the molar ratio of Mn to MnO₂ is 3:3. The moles of MnO₂ are 3/3 × 1.9 × 10² mol = 1.9 × 10² mol
In Step 1, the real yield of MnO₂ is 1.9 × 10² mol and the percent yield is 65%. The theoretical yield is:
1.9 × 10² mol (Real) × (100 mol (Theoretical) / 65 mol (Real)) = 2.9 × 10² mol
According to Step 1, the molar ratio of MnO₂ to MnCO₃ is 2:2. The moles of MnCO₃ are 2/2 × 2.9 × 10² mol = 2.9 × 10² mol
The molar mass of MnCO₃ is 114.95 g/mol. The mass of MnCO₃ represented by 2.9 × 10² mol is:
2.9 × 10² mol × (114.95 g/mol) = 3.3 × 10⁴ g = 33 kg
Ответ:
Answer : The mass of required are, 35 kg
required are, 35 kg
Explanation :
First we have to calculate the mass of .
.
The first step balanced chemical reaction is:
Molar mass of = 115 g/mole
= 115 g/mole
Molar mass of = 87 g/mole
= 87 g/mole
Let the mass of be, 'x' grams.
be, 'x' grams.
From the balanced reaction, we conclude that
As, of
of  react to give
react to give  of
of 
So, of
of  react to give
react to give  of
of 
And as we are given that the yield produced from the first step is, 65 % that means,
The mass of obtained = 0.4542x g
obtained = 0.4542x g
Now we have to calculate the mass of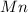 .
.
The second step balanced chemical reaction is:
Molar mass of = 87 g/mole
= 87 g/mole
Molar mass of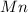 = 55 g/mole
= 55 g/mole
From the balanced reaction, we conclude that
As, of
of  react to give
react to give 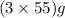 of
of 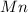
So, of
of  react to give
react to give  of
of 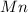
And as we are given that the yield produced from the second step is, 80 % that means,
The mass of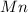 obtained = 0.2296x g
obtained = 0.2296x g
The given mass of Mn = 8.0 kg = 8000 g (1 kg = 1000 g)
So, 0.2296x = 8000
x = 34843.20 g = 34.84 kg = 35 kg
Therefore, the mass of required are, 35 kg
required are, 35 kg
Ответ: