justhereforanswers13
23.09.2019 •
Chemistry
There are three naturally occurring isotopes of the hypothetical element hilarium 45hi, 46hi, and 48hi. the percentages of these isotopes are the following: hilarium natural isotopes abundance 45hi 18.3% 46hi 34.5% 48hi 47.2% you can assume that the atomic masses are exactly equal to the mass number. calculate the weight of "naturally" occurring hilarium and report it as you would for any other naturally occurring element. answer in units of g/mol. your answer must be within ± 0.005%
Solved
Show answers
More tips
- F Family and Home Protect Your Home or Apartment from Pesky Ants...
- O Other What is a Disk Emulsifier and How Does it Work?...
- H Health and Medicine How to Calm Your Nerves? Expert Tips That Actually Work...
- A Animals and plants 5 Tips for Taking Care of Yews to Keep Them Green and Beautiful...
- S Sport How to wrap boxing hand wraps? Everything you need to know!...
- F Food and Cooking 10 Reasons Why You Should Avoid Giving Re-Gifts: An Informative Guide...
- F Family and Home Tender Care for Your Parquet: Is it Possible to Clean Parquet?...
- S Style and Beauty How Are Eyelash Extensions Applied? All Your Questions Answered...
- F Food and Cooking 10 Tips for Proper Sushi Consumption...
- S Style and Beauty Learn how to tie a keffiyeh on your head like a pro...
Answers on questions: Chemistry
- C Chemistry Keep your work area neal and...
- C Chemistry If 75% of the isotopes of an element have a mass of 45.0 amu and 25% of the isotopes have a mass of 47.0 amu, what is the atomic mass of the element?...
- C Chemistry select a field of study within environmental science describe how a professional in that field would contribute to the environmental study of a lake...
- C Chemistry How many atoms is in 1 molacule of uranium?...
- C Chemistry What mass of iron hydroxide could be produced when a solution of sodium hydroxide containing 11.25g of hydroxide ions reacts with a solution containing excess Fe³⁺ ions according...
- C Chemistry Calculate the density of a sample of one mole of ammonia gas (NH3) at 793 mmHg and -9°C...
- M Mathematics James started an apple orchard with the trees he already had and then planted 25 more. Now his orchard contains 44 trees. How many trees did James start out with?...
- E English Please help me with this:(...
- E English The following passage is an example of what? A. Simile B. Metaphor C. Hyperbole D. Synecdoche I prize thy love more than whole mines of gold, Or all the riches that the East doth...
- B Biology “My group believes that Elisa has/does not have ___. I think that she does/does not have the ___ condition because . . .” (Then, explain how molecules move through the body when...

Ответ:
46.761g/mol
Explanation:
Given parameters:
Element = Hilarium , Hi
Isotopes: Hi- 45, Hi-46 and Hi- 48
Natural abundance of Hi-45 = 18.3%
Hi-46 = 34.5%
Hi-48 = 47.2%
Unknown:
Atomic weight of naturally occurring Hilarium = ?
Solution:
Isotopes have been studied extensively by mass spectrometry. The method is used to determine the proportion/percentage/fraction by which each of the isotopes of an element occurs in nature. The proportion is called geonormal abundance. From this we can calculate the atomic weight of an element.
We can use the expression below to find this value:
Atomic weight = m₄₅α₄₅ + m₄₆α₄₆ + m₄₈α₄₈
m is the atomic mass of each isotope and α is the abundance
Atomic weight = (45 x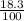 ) + (46 x
) + (46 x 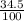 ) + (48 x
) + (48 x 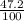 )
)
Atomic weight of Hi = 8.235 + 15.870 + 22.656 = 46.761g/mol
Ответ: