babbity2009
19.09.2021 •
Chemistry
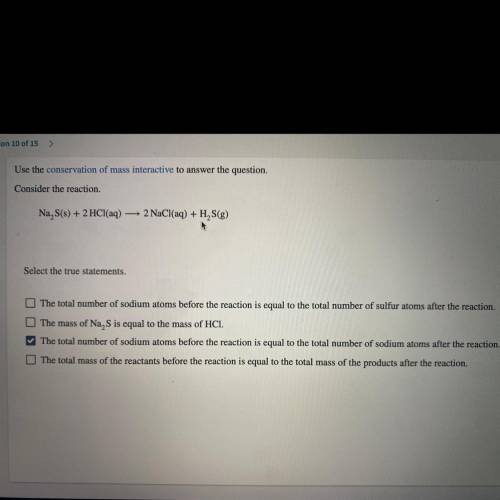
Use the conservation of mass interactive to answer the question.
Consider the reaction.
Na S(s) + 2 HCl(aq) — 2 NaCl(aq) + H2S(g)
*Select the true statements.*
A. The total number of sodium atoms before the reaction is equal to the total number of sulfur atoms after the reaction.
B. The mass of Na, S is equal to the mass of HCI.
C. The total number of sodium atoms before the reaction is equal to the
total number of sodium atoms after the reaction.
D. The total mass of the reactants before the va reaction is equal to the total mass of the products after the reaction.
Solved
Show answers
More tips
Answers on questions: Chemistry
- C Chemistry drag each statement to the correct location match each statement to the type of behavior it describes....
- M Mathematics The force of an acting on an object acceleration is 63N results in an acceleration of 9m/s if an object acceleration becomes 5 m/s what is the force...
- E English While proofreading a myth, the writer should check for select the best answer from the choices provided.a) correct capitalization in sentences b) development of conflict...
- G Geography What is the smallest amount of time that can pass between a lunar and solar eclipse...
- M Mathematics Ms. Sperlak bakes 3 1/2 pies in 20 minutes. At this rate how many pies can she bake in 1 hour? 2 hours? (This is math)...

Ответ: