What is the energy of a mole of photons that have a wavelength of 745 nm? (h = 6.626 × 10⁻³⁴ J • s and c = 3.00 × 10⁸ m/s) What is the wavelength of a photon if the energy is 7.03 × 10⁻¹⁹ J? (h = 6.626 × 10⁻³⁴ J • s)
Solved
Show answers
More tips
- H Health and Medicine 10 Ways to Cleanse Your Colon and Improve Your Health...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry How does a tornado form?...
- C Chemistry DDT is a pesticide That was once widely used to control agricultural pests in mosquitoes how ever this Petra style caused the egg shells of certain birds including brown pelican...
- C Chemistry Which molecules are pure elements?...
- C Chemistry List the ways in which tadpole and frogs differ from each other?...
- M Mathematics A family went out to eat. Below is the number of coen dogs tbat each of the four people ate. Find the mean absolute deviation (mad) of the data set....
- M Mathematics To have academic integrity means that you A. complete your work honestly and only submit work that is your own B. submit work that your friends did for you C. complete your work...
- G Geography Science When viewed from Earth, the sun appears larger than any other star in the sky , which of these models explains why? A. A snowball tends to get larger as it rolls downhill...
- E English 7. How is the style of a piece of writing related to its tone? A. Style and tone are unrelated aspects of writing. B. Every piece of writing has a certain style, but only formal...
- M Mathematics Captain Patel uses a wheel to steer his boat. The circumference of the wheel is 116.2 inches. How tall is the wheel? *Use 3.14 for pi*...
- S Social Studies What poverty do to people ?...

Ответ:
The energy carried by a single photon of 535 nm light is 3.71 × 10−19 J.
The equation used to find the energy in a mole of photons is E= hc/lambda where h is Planck's constant, c is the speed of light and is the wavelength of light.
Ответ:
Option B is correct.
Step-by-step explanation:
Diagram of given scenario shown below.
Given that,
Diameter of cylinder is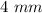 .
.
Height of cylinder is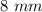 .
.
So, Radius of cylinder is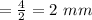
Volume of cylinder = {where,
{where, 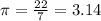 ,
,  is the radius
is the radius
of cylinder and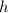 is height of cylinder}
is height of cylinder}
Substituting the values we get,
Volume of cylinder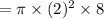
Therefore, Required volume of Cylinder rounding it to the nearest tenth ≈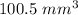 .
.
Hence, option B is correct.