When 4.008 g of sulfur is burned completely in abundant air, it combines with oxygen to form a single gaseous product with a total mass of 10.008 g. The mass percent of sulfur in the product is % (2 dec places). The mass percent of oxygen in the product is % (2 dec places).
Solved
Show answers
More tips
- P Philosophy Agnosticism: Opinion or Belief?...
- S Style and Beauty How to choose the best mascara for your eyelashes...
- F Food and Cooking Discover Delicious Recipes You Can Make with Ground Meat...
- C Computers and Internet Google Search Tips and Tricks: Everything You Need to Know...
- S Science and Technology Why is there no gravity on other planets?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
Answers on questions: Chemistry
- C Chemistry ¿Qué carga circula por una pila, que es capaz de entregar 3 A durante 100 segundos? *...
- C Chemistry Explain how specialized structures found in multiflora rose support its reproduction and spreading/dispersal throughout ecosystems in the United States. Use evidence and...
- C Chemistry What is the amount of a solute (in mol) needed to make 0.250 L of an NaF solution with a concentration of 1.25 M?...
- C Chemistry Which of the following is not an example of an externality? a. acidic by-products of fossil fuel combustion that produce acid rain. b. carbon dioxide from energy generation...
- C Chemistry which of the following statements would be correct if one mole of hydrogen was used in this reaction: 2H2 + O2 = 2H2O...
- C Chemistry Will mark brainliest for answer with full calculations. Please HELP!! Calculate the solubility of iron(II) carbonate at 25°C. The Ksp of FeCO3(s) is 3.5 x 10-11 at 25°C....
- C Chemistry What is the chemical symbol for sodium? a.na b.s c.na d.so?...
- C Chemistry Need i am going to fail a chemical reaction has an increase in entropy and a decrease in enthalpy. what is known about the spontaneity of the reaction? spontaneous at...
- C Chemistry Chlorine has two isotopes. the isotope with a mass of 34.969 amu has a relative abundance of 75.77%. the isotope with a mass of 36.966 amu has a relative abundance of...
- C Chemistry Which statement about isotopes is correct? isotopes are different elements with different numbers of protons but the same number of neutrons.isotopes are the same element...

Ответ:
The mass percent of sulfur and oxygen in the compound is 40.05 % and 59.95 % respectively
Explanation:
To calculate the mass percentage of element, we use the equation:
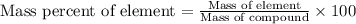
For sulfur:Mass of sulfur = 4.008 g
Mass of compound = 10.008 g
Putting values in above equation, we get:
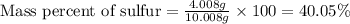
For oxygen:Mass of oxygen = [10.008 - 4.008] = 6 g
Mass of compound = 10.008 g
Putting values in above equation, we get:
Hence, the mass percent of sulfur and oxygen in the compound is 40.05 % and 59.95 %
Ответ:
Chinua Achebe wrote about the way of life in Nigeria that avoided stereotyping African identity, and at the same time keeping a critical viewpoint of the actions humans take that lead to their downfall. The aim of Achebe´s fiction is to take normal everyday passions that are common to all human beings and expose them without falling prey to over-simplified stereotypes, which tend to dehumanize the subject of the story. In ´Things Fall Apart´, for example, the plight of the main character is described without resorting to any sort of value judgements on the village´s strict and un-western system of justice.