OnlyaBurden
07.12.2021 •
Chemistry
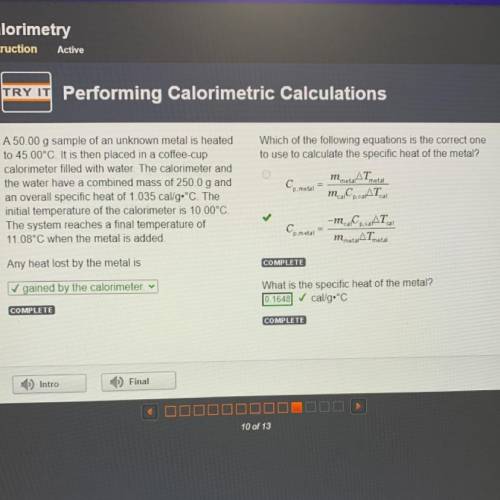
Which of the following equations is the correct one
to use to calculate the specific heat of the metal?
AT
Cp.metal
m.Co.AT
metal
A 50.00 g sample of an unknown metal is heated
to 45.00°C. It is then placed in a coffee-cup
calorimeter filled with water. The calorimeter and
the water have a combined mass of 250.0 g and
an overall specific heat of 1.035 cal/g•°C. The
initial temperature of the calorimeter is 10.00°C.
The system reaches a final temperature of
11.08°C when the metal is added.
metal
cal
Ce metal
-m.C.CAT
m metarATmetal
COMPLETE
Any heat lost by the metal is
✓ gained by the calorimeter v
What is the specific heat of the metal?
cal/g.°C
COMPLETE
DONE
Solved
Show answers
More tips
- A Art and Culture Attention, the Final Episode of Margo is Almost Here!...
- W Work and Career How to Start Your Own Business: Tips and Recommendations...
- S Society and Politics 10 Tips for Boosting Your Self-Esteem...
- C Computers and Internet How to Create a Folder on Your iPhone?...
- G Goods and services How to sew a ribbon: Tips for beginners...
- F Food and Cooking How to Make Mayonnaise at Home? Secrets of Homemade Mayonnaise...
- C Computers and Internet Which Phone is Best for Internet Surfing?...
- F Food and Cooking Everything You Need to Know About Pasta...
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
Answers on questions: Chemistry
- C Chemistry What is the oxidation half-reaction for mg(s) + zncl2(aq) mgcl2(aq) + zn(s)? a. zn(s) -- zn^2+ + 2e- b. mg(s) -- mg2+ + 2e- c. zn^2+ + 2e- -- zn(s) d. mg^2+ + 2e- -- mg(s)...
- C Chemistry Example best shows that the chemistry of water is to plants? water’s polarity produces a high density, which allows water to move to the leaves. water’s bent shape causes...
- C Chemistry If a liquid sample of naphthalene is heated and remains at 218 degrees c until it is completely vaporized, you know that 218 degrees c is the of naphthalene a) freezing...
- C Chemistry What solute particles are present in an aqueous solution of ch3coch3?...
- C Chemistry Please help, Question 1 In the electron configuration for Helium, 182, what does the 1 represent? (TEKS 6D.1) 1 Answer A Subshell B Energy level 2 С Number of electrons...
- C Chemistry Question 4 (1 point) Very high wispy clouds formed mostly of ice crystals. Stratus Cirrus Cumulous...
- C Chemistry Every element in the first period has shell for its . Every element in the second period has for its . See the pattern?...
- M Mathematics A number is divisible by both 3 and 9. A number is missing. 1 5 5 _ 7 a. Both 0 and 3 b. Both 0 and 9 c. Only 3 d. Only 9...
- M Mathematics 1. A line goes through the points (4, 5) and (2, -6). Write the equation of the line in point-slope form. Show your work for full credit. (Show how you went from those...
- M Mathematics What is the aswer ??????...

Ответ:
What is the specific heat of a substance whose mass is 7 grams, if it absorbs 180 calories to go from 10 degrees Celsius to 85 degrees Celsius
0.343cal/g°C
Explanation:
Given parameters:
Mass of substance = 7g
Quantity of heat absorbed = 180calories
Initial temperature = 10°C
Final temperature = 85°C
Unknown:
Specific heat of the substance = ?
Solution:
To solve this problem, the specific heat of a substance is the amount of heat needed to raise the temperature of a unit mass of that substance by 1°C.
Mathematically;
C =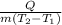
C is the specific heat
Q is the amount of heat
m is the mass
T is the temperature
1 and 2 are the initial and final states
Now, insert the parameters and solve;
C = = 0.343cal/g°C
= 0.343cal/g°C