silviamgarcia
24.07.2019 •
Chemistry
You have two 466.0 ml aqueous solutions. solution a is a solution of silver nitrate, and solution b is a solution of potassium chromate. the masses of the solutes in each of the solutions are the same. when the solutions are added together, a blood-red precipitate forms. after the reaction has gone to completion, you dry the solid and find that it has a mass of 331.8 g. (a) calculate the concentration of the potassium ions in the original potassium chromate solution.(b) calculate the concentration of the chromate ions in the final solution
Solved
Show answers
More tips
- S Style and Beauty How to Grow Hair Faster: Real Methods and Advice...
- F Family and Home How to Remove Fading from Clothes: Tips and Tricks...
- F Food and Cooking How to Make Polendwitsa at Home?...
- F Family and Home Parents or Environment: Who Has the Most Influence on a Child s Upbringing?...
- P Philosophy Unbelievable stories of encounters with otherworldly forces...
- L Leisure and Entertainment How to Choose the Perfect Gift for Men on February 23rd?...
- H Health and Medicine How to Treat Whooping Cough in Children?...
- H Health and Medicine Simple Ways to Lower Cholesterol in the Blood: Tips and Tricks...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
Answers on questions: Chemistry
- C Chemistry Glass breaking is an example of: chemical change chemical properties temperature change physical change...
- C Chemistry Which chemical is the limiting reagent for the entire series of reactions? why?...
- C Chemistry Read the prompt and study the diagram. What cause and effect prediction can be used to test the assumptions in the model? A) with more sunlight the plant will produce less...
- C Chemistry I need help with these 2 please help...
- C Chemistry Potassium chloride and lead (ii) nitrate undergo a double replacement reaction. Predict the formulae of the two products of the reaction....
- C Chemistry Marita is analyzing the population of various songbirds in her neighborhood. She estimates how the population is increasing by and decreasing by as different birds enter...
- M Mathematics Find a recipe un a book or online that includes the amout of salt as a fraction. model how to find the amount of salt you need when the recipe is doubled...
- C Chemistry The particles in which sample of licl(s) have the same average kinetic energy as the particles in a 2.0-mole sample of h2o(l) at 25°c? (1) 1.0 mol at 75°c (2) 2.0 mol at...
- M Mathematics What is the relation between the variables in the equation x^4/y=7?...
- C Chemistry What product(s) are expected in the ethoxide-promoted β-elimination reaction of 2-bromo-2,3-dimethylbutane? omit ions, salts, and ethanol from your response....

Ответ:
The concentration of the potassium ions in the original potassium chromate solution is 4.2927 mol/L.
The concentration of the chromate ions in the final solution is 1.0731 mol/L.
Explanation:
Volume of solution A i.e. solution of silver nitrate = 466.0 mL = 0.466 L
Volume of solution B i.e. solution of potassium chromate = 466.0 mL = 0.466 L
Moles of silver chromate =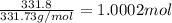
According to reaction , 1 mol of silver chromate is produce from 2 moles of silver nitrate.
Then, 1.0002 moles of silver chromate will be formed from:
According to reaction , 1 mol of silver chromate is produce from 1 mole of potassium chromate.
Then, 1.0002 moles of silver chromate will be formed from:
a) The concentration of the potassium ions in the original potassium chromate solution.
Volume of the original solution = 0.466 L
1 mol of potassium chromate dissociates into 2mol of potassium ions and 1 mol of chromate ions:
Moles of potassium ions = 2 × 1.0002 mol = 2.0004 mol
b) The concentration of the chromate ions in the final solution
Volume of the final solution = 0.466 L + 0.466 L
Moles of chromate ions = 1 × 1.0002 mol = 1.0002 mol
Ответ:
Answer
I dont know
Explanation:
do it yourself