A2.64-kg copper part, initially at 400 k, is plunged into a tank containing 4 kg of liquid water, initially at 300 k. the copper part and water can be modeled as incompressible with specific heats 0.385 kj/kg ? k and 4.2 kj/kg k, respectively. for the copper part and water as the system, determine (a) the final equilibrium temperature, in k, and (b) the amount of entropy produced within the tank, in kj/k. ignore heat transfer between the system and its surrounding
Solved
Show answers
More tips
- C Computers and Internet Are there special gaming mice?...
- L Leisure and Entertainment When will Maslenitsa start?...
- F Food and Cooking Discovering the Mysterious Fruit of Feijoa...
- B Business and Finance How to Open an Online Store? A Detailed Guide for Beginners...
- W Work and Career How to Write a Resume That Catches the Employer s Attention?...
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
Answers on questions: Physics
- P Physics A stone is thrown straight up in the air. If g represents the acceleration due to gravity, which one of the following statments about the stone is false? When the stone reaches...
- P Physics The air contained water that froze at 0 °C The change in internal energy of the water as it froze was 0.70 kJ The specific latent heat of fusion of water is 330 kJ/kg Calculate...
- P Physics Help! Need answers for #1-5....
- P Physics Which is an example of a transverse wave? A. an ultrasound that transmits sound waves B. crowd doing the wave at a sporting event C. the waves formed by an earthquake D. the...
- P Physics Why do black holes exist?...
- P Physics A small ball of mass m = 5 kg is suspended froma string of length L = 5 m. The ball revolveswith constant speed v in a horizontal circle ofradius r = 2 m. Find an expression...
- P Physics If the wave above takes 6 seconds to get from beginning to end, what is the speed of the wave?...
- P Physics In the diagram, q1 and q2 are both +5.00*10^-8 C. What is the electric field at point P? Include a + or - sign to indicate the direction....
- P Physics 54 j of work was done on a 45 n object. how far did the object move? a. 0.8 m b. 1.2 m c. 4.5 m d. 9.0 m...
- P Physics Will mark brainliest which class of lever is a seesaw? what are the parts of a seesaw s lever class? describe each one. enter your answer in the space provided....

Ответ:
a)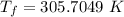
b)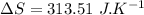
Explanation:
Given:
mass of copper,a)
∵No heat is lost in the environment and the heat is transferred only between the two bodies:
Heat rejected by the copper = heat absorbed by the water
b)
Now the amount of heat transfer:
∴Entropy change
Ответ: