kwoolfe59006
16.10.2019 •
Physics
An atom of neon has a radius rne = 38. pm and an average speed in the gas phase at 25°c of 350./ms. suppose the speed of a neon atom at 25°c has been measured to within 0.010%. calculate the smallest possible length of box inside of which the atom could be known to be located with certainty. write your answer as a multiple of rne and round it to 2 significant figures. for example, if the smallest box the atom could be in turns out to be 42.0 times the radius of an atom of neon, you would enter "42.rne" as your answer.
Solved
Show answers
More tips
- S Science and Technology Why is there no gravity on other planets?...
- F Food and Cooking Deflope: What is it and how does it work?...
- B Business and Finance How to Create a Business Plan? Your Ultimate Guide...
- F Food and Cooking Unusually Delicious Shashlik - Follow the Etiquette of Proper Preparation!...
- C Computers and Internet Make Easy Accessible Screenshots on iPad in Just a Few Minutes...
- T Travel and tourism Lost in the Catacombs: What to Do?...
- F Family and Home Protect Your Home or Apartment from Pesky Ants...
- H Health and Medicine How to Treat Styes: Causes, Symptoms, and Home Remedies...
- L Legal consultation What Documents Are Required for a Russian Passport?...
- F Family and Home How to Properly Use a Water Level?...
Answers on questions: Physics
- P Physics Electromagnetic Induction Quiz Active TIME REMAINING 39:37 Which change will cause an increase in the electric current produced through electromagnetic induction? O using more...
- H History Under the articles of confedration, the national government could regulate the trade of the different states. true or false...
- M Mathematics The perimeter of this rectangle is 30 units. How long is the longer side?...
- M Mathematics PLEASE HELP WILL MARK U BRAINLIEST...
- B Biology Carbon monoxide is a poisonous gas because it competes with the gas that binds to hemoglobin in red blood cells. Which of the following would be most directly affected by carbon...

Ответ:
1.2* 10³ rNe.
Explanation:
Given speed of neon=350 m/s
Un-certainity in speed= (0.01/100) *350 =0.035 m/s
As per heisenberg uncertainity principle
Δx*mΔv ≥ ..................(1)
..................(1)
mass of neon atom =
substituating the values in eq. (1)
Δx =4.49*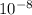 m
m
In terms of rNe i.e 38 pm= 38*
Δx=
=0.118* * (rNe)
* (rNe)
=1.18*10³ rN
= 1.2* 10³ rNe.
Ответ:
P since without a host the parasite won't be able to survive they would start decreasing as well but if the hosts were no more then they would go extinct. But since it is just decreasing then it should be P