KWffrd6661
24.03.2021 •
Chemistry
5. in
(3 Points)
Which of the following has the most number of atoms (all in Group 1A):
(Circle the correct answer)
5 g Li (Lithium)
b. 20 g Na (Sodium) 2.1
80 g Rb (Rubidium) 1.4
125 g Cs (Cesium)
All have the same number of atoms
C
a.
d.
f.
45 g K (Potassium)
e.
ii.
(2 points)
Which of the following has the least number of molecules:
(Circle the correct answer)
1 mole of N2 gas
b.
1 mole of O2 gas
1 mole of Cl2 gas
1 mole of Br2 gas
All have the same number of molecules
C.
1 mole of F2 gas
ro ou
e.
Solved
Show answers
More tips
- F Food and Cooking When is Easter in 2011?...
- L Leisure and Entertainment Scrapbooking: What is it and Why is it Becoming More Popular?...
- H Horoscopes, Magic, Divination How to Cast a Love Spell on a Guy? Guide for Guys...
- F Family and Home How to Sew Curtain Tapes: Best Tips from Professionals...
- S Style and Beauty How are artificial nails removed?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- F Food and Cooking The Disease That Haunted Abraham Lincoln...
- C Computers and Internet How to Get Rid of Windows Genuine Check?...
- H Health and Medicine How to perform artificial respiration?...
- S Style and Beauty Tricks and Tips: How to Get Rid of Freckles...
Answers on questions: Chemistry
- M Mathematics Please I need help I’ll give you Brinley you need to put them in standard form...
- M Mathematics Write the equation from par in slope-intercept form use function notation use the linear function to predict the traveled distance when the lapse of time is 20...
- H Health Research participants preferred specific vacation destinations that they had simply imagined themselves experiencing. if the imagination process followed consumption of...
- B Business How can managers overcome obstacles to diversity such as mistrust and tension, stereotyping, and communication problems?...

Ответ:
Answer is in the photo. I can't attach it here, but I uploaded it to a file hosting. link below! Good Luck!
tinyurl.com/wpazsebu
Ответ:
Explanation:
As molar mass of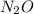 is 44.013 g/mol and it is given that its mass is 0.195 g.
is 44.013 g/mol and it is given that its mass is 0.195 g.
Therefore, calculate its number of moles as follows.
No. of moles =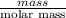
=
= 0.0044 mol
Here, in 1 mole of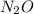 there is 2 mole of N. Thus, moles of N present in it is calculated as follows.
there is 2 mole of N. Thus, moles of N present in it is calculated as follows.
= 0.0088 mol
Thus, we can conclude that in 0.195 g of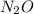 there are 0.0088 mol of N.
there are 0.0088 mol of N.