alystidham772
08.03.2021 •
Chemistry
A mixture of 75 mole% methane and 25 mole% hydrogen is burned with 25% excess air. Fractional conversions of 90% of the methane and 85% of the hydrogen are achieved; of the methane that reacts, 95% reacts to form CO2 and the balance reacts to form CO. The hot combustion product gas passes through a boiler in which heat transferred from the gas converts boiler feedwater into steam.
Required:
a. Calculate the concentration of CO (ppm) in the stack gas.
b. The CO in the stack gas is a pollutant. Its concentration can be decreased by increasing the percent excess air fed to the furnace. Think of at least two costs of doing so. (Hint: The heat released by the combustion goes into heating the combustion products; the higher the combustion product temperature, the more steam is produced.)
Solved
Show answers
More tips
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
Answers on questions: Chemistry
- C Chemistry 1. for dry air at 1. atm pressure, the densities at –50°c, 0°c, and 69°c are 1.5826 g dm–3 , 1.2929 g dm–3, and 1.0322 g dm–3, respectively. a) assume a sample of mass 1000 g, and...
- C Chemistry In the supplemental information, figure s2 shows the x-ray powder diffraction patterns for the complete yin1-xmnxo3series. explain how these data support the conclusion that a complete...
- C Chemistry Complete the sentence. Increasing the of a solvent increases the solubility of the solute....
- C Chemistry After each step in a dichotomous key, the questions get . Which phrase best completes the sentence? 1. more specific 2.more difficult 3.less specific 4.less difficult...
- C Chemistry Which subatomic particle orbits the nucleus? the electron the neutron the proton please help me first to answer i ll give brainlist!...
- C Chemistry FeO. Ti02the minerals from it...
- C Chemistry Energy can be transformed from one form to another. The diagram shows one such process. Which energy transformation is represented in the diagram? Nucleus O nuclear to thermal and...
- C Chemistry Complete the missing information in the reactions. Then, label each reaction as Alpha (a) or Beta (B) decay. 14 0 N 9 Type: : n: p: n: p: n: P: 238 U 10. 92 234 Th 90 + Type: : n:...
- C Chemistry Tell a embarrassing story that happened to you or that you have seen at school...
- C Chemistry Where we get our electricity?...

Ответ:
Solution :
Consider a mixture of methane and hydrogen.
Take the basis as 100 moles of the mixture.
The mixture contains 75% of methane and 25% of hydrogen by mole and it is burned with 25% in excess air.
Moles of methane = 0.75 x 100
Moles of hydrogen = 0.25 x 100
The chemical reactions involved during the reaction are :
The fractional conversion of methane is 90%
Number of moles of methane burned during the reaction is = 0.9 x 75
= 67.5
Moles of methane leaving = initial moles of methane - moles of methane burned
= 75 - 67.5
= 7.5 moles
Fractional conversion of hydrogen is 85%
The number of moles of hydrogen burned during the reaction is = 0.85 x 25
= 21.25
Moles of hydrogen leaving = initial moles of hydrogen - moles of hydrogen burned
= 25 - 21.25
= 3.75 moles
Methane undergoing complete combustion is 95%.
= 64.125 moles
= 3.375 moles
Oxygen required for the reaction is as follows :
From reaction 1, 1 mole of the methane requires 2 moles of oxygen for the complete combustion.
Hence, oxygen required is = 2 x 75
= 150 moles
From reaction 3, 1 mole of the hydrogen requires 0.5 moles of oxygen for the complete combustion.
Hence, oxygen required is = 0.5 x 25
= 12.5 moles
Therefore, total oxygen is = 150 + 12.5 = 162.5 moles
Air is 25% excess.
SO, total oxygen supply = 162.5 x 1.25 = 203.125 moles
Amount of nitrogen =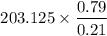
= 764.136 moles
Total oxygen consumed = oxygen consumed in reaction 1 + oxygen consumed in reaction 2 + oxygen consumed in reaction 3
Oxygen consumed in reaction 1 :
1 mole of methane requires 2 moles of oxygen for complete combustion
= 2 x 64.125
= 128.25 moles
1 mole of methane requires 1.5 moles of oxygen for partial combustion
= 1.5 x 3.375
= 5.0625 moles
From reaction 3, 1 mole of hydrogen requires 0.5 moles of oxygen
= 0.5 x 21.25
= 10.625 moles.
Total oxygen consumed = 128.25 + 5.0625 + 10.625
= 143.9375 moles
Total amount of steam = amount of steam in reaction 1 + amount of steam in reaction 2 + amount of steam in reaction 3
Amount of steam in reaction 1 = 2 x 64.125 = 128.25 moles
Amount of steam in reaction 2 = 2 x 3.375 = 6.75 moles
Amount of steam in reaction 3 = 21.25 moles
Total amount of steam = 128.25 + 6.75 + 21.25
= 156.25 moles
The composition of stack gases are as follows :
Number of moles of carbon dioxide = 64.125 moles
Number of moles of carbon dioxide = 3.375 moles
Number of moles of methane = 7.5 moles
Number of moles of steam = 156.25 moles
Number of moles of nitrogen = 764.136 moles
Number of moles of unused oxygen = 59.1875 moles
Number of moles of unused hydrogen = 3.75 moles
Total number of moles of stack gas
= 64.125+3.375+7.5+156.25+764.136+59.1875+3.75
= 1058.32 moles
Concentration of carbon monoxide in the stack gases is
= 3189 ppm
b). The amount of carbon monoxide in the stack gas can be decreased by increasing the amount of the excess air. As the amount of the excess air increases, the amount of the unused oxygen and nitrogen in the stack gases will increase and the concentration of CO will decrease in the stack gas.
Ответ:
The correct answer is 1.61 L.
Explanation:
Based on the given information, 3.712 grams of Mg reacts with 104.2 ml of 1.385 mol per L HCl at SATP, there is a need to find the amount of hydrogen gas produced in liters.
The chemical reaction taking place in the given case is,
Mg + 2HCl = MgCl2 + H2
The reacting moles of each reactants is,
Moles of Mg = 3.712 g/24.305 g = 0.153 moles
Moles of HCl = 1.385 mol/L * 0.1042 L = 0.144 moles
From the reaction it is clear that Mg and HCl are present in 1:2 molar ratio. Therefore, 0.153 moles of Mg can completely react with 0.306 moles of HCl. However, the moles of HCl obtained in the given case is only 0.144 moles, thus, HCl is a limiting reactant.
Now the moles of hydrogen produced is,
n = 0.144 moles of HCl * (1 mole H2/2 mol HCl) = 0.072 moles
Finally to find the liters of hydrogen gas produced, the ideal gas equation is used, that is, PV = nRT
At STAP, the value of T is 273 K and pressure is 1 atm, the value of R is 0.082 atm.L/mol.L. Now putting the values we get,
PV = nRT
V = nRT/P
V = 0.072 mol * 0.082 atm.L/mol.L*273/ 1 atm
V = 1.61 L