jessicascott120305
02.07.2019 •
Chemistry
Combine the two half-reactions that give the spontaneous cell reaction with the smallest e∘. fe2+(aq)+2e−→fe(s) e∘=−0.45v i2(s)+2e−→2i−(aq) e∘=0.54v cu2+(aq)+2e−→cu(s) e∘=0.34v
Solved
Show answers
More tips
- S Sport How to Pump Your Chest Muscle? Secrets of Training...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- S Style and Beauty How are artificial nails removed?...
- F Family and Home How to Sew Curtain Tapes: Best Tips from Professionals...
- H Horoscopes, Magic, Divination How to Cast a Love Spell on a Guy? Guide for Guys...
- F Family and Home How to Properly Use a Water Level?...
- L Legal consultation What Documents Are Required for a Russian Passport?...
- H Health and Medicine How to Treat Styes: Causes, Symptoms, and Home Remedies...
- F Family and Home Protect Your Home or Apartment from Pesky Ants...
Answers on questions: Chemistry
- C Chemistry Consider a monatomic gas of particles each with mass m. what is vx,rms=⟨v2x⟩−−−−√, the root mean square (rms) of the x component of velocity of the gas particles if the gas is at...
- M Mathematics What is the locus of points in space that are r units from point A...
- H History Has anyone taken the UNIT TEST: Regional Civilizations and Cultures - Part 1 if you did please give me the answers!! I did the quiz but I m not 100% sure about my answers. WILL MARK...
- P Physics How would you best use the knowledge and skills learned in social dancing to enhance your community’s fitness?...

Ответ:
The spontaneous cell reaction having smallest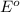 is
is 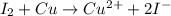
Explanation:
We are given:
The substance having highest positive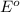 potential will always get reduced and will undergo reduction reaction. Here, iodine will always undergo reduction reaction, then copper and then iron.
potential will always get reduced and will undergo reduction reaction. Here, iodine will always undergo reduction reaction, then copper and then iron.
The equation used to calculate electrode potential of the cell is:
The combination of the cell reactions follows:
Case 1:Here, iodine is getting reduced and iron is getting oxidized.
The cell equation follows:
Oxidation half reaction:

Reduction half reaction: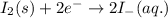

Thus, this cell will not give the spontaneous cell reaction with smallest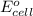
Case 2:Here, iodine is getting reduced and copper is getting oxidized.
The cell equation follows:
Oxidation half reaction: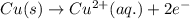
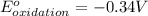
Reduction half reaction: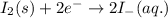

Thus, this cell will give the spontaneous cell reaction with smallest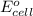
Case 3:Here, copper is getting reduced and iron is getting oxidized.
The cell equation follows:
Oxidation half reaction:

Reduction half reaction: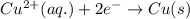
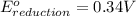
Thus, this cell will not give the spontaneous cell reaction with smallest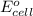
Hence, the spontaneous cell reaction having smallest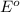 is
is 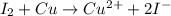
Ответ: