Consider the half reactions below for a chemical reaction.
mg— mg2(aq) + 2e-
2h* (aq) + 2e--> h2(g)
what is the overall equation for this chemical reaction?
mg(s)+2h+ (aq) — mg2+ (aq) + h2(
mg(s) + 2h+ (aq) —> mg2+ (aq) + 2e-
mg2+ (aq)+h2(9)—> mg(s)+ 2h+ (aq)
mg(s)+2h--> mg(aq) + h2(g)
Solved
Show answers
More tips
- C Computers and Internet How to Choose a Monitor?...
- H Horoscopes, Magic, Divination Where Did Tarot Cards Come From?...
- S Style and Beauty How to Make Your Lips Fuller? Ideas and Tips for Beautiful Lips...
- S Style and Beauty How are artificial nails removed?...
- F Family and Home How to Sew Curtain Tapes: Best Tips from Professionals...
- H Horoscopes, Magic, Divination How to Cast a Love Spell on a Guy? Guide for Guys...
- F Family and Home How to Properly Use a Water Level?...
- L Legal consultation What Documents Are Required for a Russian Passport?...
- H Health and Medicine How to Treat Styes: Causes, Symptoms, and Home Remedies...
- F Family and Home Protect Your Home or Apartment from Pesky Ants...
Answers on questions: Chemistry
- C Chemistry New substances with what are formed during chemical changes...
- C Chemistry Which of the following would increase the elastic force acting on that object? SELECT ALL THAT APPLY a Increasing the weight being vertically supported by a string b Moving a...
- C Chemistry Methane combusts according to the following thermochemical equation: CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH = -891 kJ If 25 g of methane combusts, how much energy is released in...
- C Chemistry 1. A group of individual of the same species that exist together at the same time Population density 2. The number of individuals making up a population Population size Carrying...
- C Chemistry Forensic chemists working for Chem Lab Burns, Inc. often work with police to help identify unknown materials found at crime scenes. Many compounds have the same chemical formula,...
- C Chemistry Help meeeeeeeeeeeee pleaase...
- P Physics Imagine that distance d is much greater than the length of the wire. intuitively, what should the magnitude of the electric field at point p be in this case? express your answer...
- M Mathematics Mixed chocolates michael and kevin want to buy chocolates. they can’t agree on whether they want chocolate– covered almonds, chocolate-covered peanuts, or chocolate- covered raisins....
- B Biology What are the steps of transcription and translation...
- C Chemistry What mass of copper has the same number of atoms as 26.98 g of aluminum? show your work for full credit! a. convert the mass of aluminum to moles of aluminum. molar mass of aluminum...

Ответ:
Explanation:
The half reactions are:
Mg → Mg²⁺
→ Mg²⁺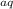 + 2e⁻
+ 2e⁻
2H⁺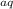 + 2e⁻ → H₂
+ 2e⁻ → H₂
The first equation is an oxidation half while the second is a reduction half. To get the overall reaction equation, we simply combine the two equations. The reactants combines at the left hand side with the products comes together at the right hand side.
Mg + 2H⁺
+ 2H⁺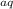 + 2e⁻ → Mg²⁺
+ 2e⁻ → Mg²⁺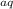 + 2e⁻ + H₂
+ 2e⁻ + H₂
Now we cancel out the species that appear on both sides:
Mg + 2H⁺
+ 2H⁺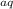 → Mg²⁺
→ Mg²⁺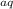 + H₂
+ H₂
Ответ:
44444444444444444
594923902394-0324q493409pExplanation: