Given these reactions, X ( s ) + 1 2 O 2 ( g ) ⟶ XO ( s ) Δ H = − 668.5 k J / m o l XCO 3 ( s ) ⟶ XO ( s ) + CO 2 ( g ) Δ H = + 384.3 k J / m o l what is Δ H for this reaction? X ( s ) + 1 2 O 2 ( g ) + CO 2 ( g ) ⟶ XCO 3 ( s )
Solved
Show answers
More tips
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Chemistry
- C Chemistry What is the oxidation state of phosphorus in the following compounds? a. phosphoric acid b. phosphorus pentoxide c. phosphine...
- C Chemistry Hey ;) butiful+hansome= me and you❤...
- C Chemistry Determine the grams in a sample of 5 mol of beryllium carbide - Be2C....
- C Chemistry A 15 kg cart filled with apples is sitting at the top of a hill that is 21 m high. The apples have a mass of 33 kg. What is the PE of the cart and the apples?...
- C Chemistry Which element is more reactive? A) Flourine B) Oxygen C)Cabron D)Boron...
- C Chemistry If an aerosol can at room temperature (20.0 degree Celsius) and a pressure of 1.0 atm is thrown in a fire. If the pressure increased to 4.10 atm, what is the temperature...
- E English Help plzzz 20 points to help...
- M Mathematics Write the letter for each equation shown in the box into the category to show whether the equation represents a linear or...
- M Mathematics The diagram shows a rectangle.0.5 cm7 cm.Four of these rectangles are put together as shown.Calculate the shaded area....
- M Mathematics Find the approximate volume of this prism. 62 m3 44 m3 72 m3 108 m3...

Ответ:
The for the reaction is -1052.8 kJ.
for the reaction is -1052.8 kJ.
Explanation:
Hess’s law of constant heat summation states that the amount of heat absorbed or evolved in a given chemical equation remains the same whether the process occurs in one step or several steps.
According to this law, the chemical equation is treated as ordinary algebraic expressions and can be added or subtracted to yield the required equation. This means that the enthalpy change of the overall reaction is equal to the sum of the enthalpy changes of the intermediate reactions.
The given chemical reaction follows:
The intermediate balanced chemical reaction are:
(1)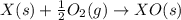

(2)

The expression for enthalpy of the reaction follows:
Putting values in above equation, we get:
Hence, the for the reaction is -1052.8 kJ.
for the reaction is -1052.8 kJ.
Ответ:
I like trains
Explanation:
5