Reebear1447
30.10.2019 •
Chemistry
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric acid, that citric acid is a monoprotic acid, and that the density of lemon juice is 1.0 g/ml, then the citric acid concentration calculates to 0.5% by mass. estimate the volume of 0.0100 m naoh required to neutralize a 3.71-g sample of lemon juice. the molar mass of citric acid is 190.12 g/mol.
Solved
Show answers
More tips
- G Goods and services Kogda zhdatt Iphone 5? The Latest News and Rumors...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
Answers on questions: Chemistry
- H Health Which provision concerns the insured s duty to provide the insurer with reasonable notice in the event of a loss?...
- M Mathematics Determine the slope-intercept form of the equation of the line parallel to y = x + 11 that passes through the point (–6, 2). y = x + =...
- E English E Imagine that you are the narrator of the story. You enter the house and after waiting a few minutes, other people begin to arrive. It is now the afternoon of the following day......
- H History What culture movement is know as one of the first american artistic and intelectual movements in the united states...

Ответ:
The volume to neutralize the sample of lemon juice is 9,76mL
Explanation:
3,71 g sample of lemon juice are:
3,71 g × = 0,01855 g of citric acid. In moles:
= 0,01855 g of citric acid. In moles:
0,01855 g × = 9,76x10⁻⁵ moles of citric acid.
= 9,76x10⁻⁵ moles of citric acid.
Assuming this acid is a monoprotic acid, the moles of NaOH required to neutralized the citric acid are 9,76x10⁻⁵ moles
As the molar concentration of NaOH is 0,0100 mol/L. The volume to neutralize the sample of lemon juice is:
9,76x10⁻⁵ moles ×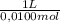 = 9,76x10⁻³L ≡ 9,76 mL
= 9,76x10⁻³L ≡ 9,76 mL
I hope it helps!
Ответ:
aight
Explanation:
aight