whitakers87
26.09.2019 •
Chemistry
Methane, ch4, reacts with i2 according to the reaction ch4(g)+i2(g)⇌ch3i(g)+hi(g)
at 630 k, kp for this reaction is 2.26×10−4. a reaction was set up at 630 k with initial partial pressures of methane of 105.1 torr and of 7.96 torr for i2.
calculate the pressure, in torr, of ch4.
express your answer to four significant figures and include the appropriate units.
calculate the pressure, in torr, of i2.
express your answer to three significant figures and include the appropriate units.
calculate the pressure, in torr, of ch3i.
express your answer to three significant figures and include the appropriate units.
calculate the pressure, in torr, of hi.
express your answer to three significant figures and include the appropriate units.
Solved
Show answers
More tips
- C Computers and Internet Where are passwords stored in Opera?...
- H Health and Medicine Simple and Effective: How to Get Rid of Cracked Heels...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
Answers on questions: Chemistry
- C Chemistry The oxyorganic compound most likely to be an acid is: c2h5oh c2h5cooh ch2o ch3oh...
- C Chemistry Fast! a p sublevel is represented by the quantum number (2 points) m subscript l = 1 m subscript l = 2 l = 1 l = 2...
- C Chemistry Which one of the following tells the physical state (matter) of an element at room temperature? a. atomic number b. the color of the chemical symbol c. atomic mass d. element...
- C Chemistry Amole is defined to of a substance....
- C Chemistry How would you name the number:100 liters? A. Dekaliter B. Hectoliter C. Centiliter D. Megaliter E. Other...
- C Chemistry Consider a cloudless day on which the sun shines down across the United States. If 2014 kJ of energy reaches a square meter( m 2) of the United States in one hour, how...
- C Chemistry What is the correct answer?...
- C Chemistry Suppose that a series of measurement have shown that the mean mass of an object is 122.4 g with a standard deviation 1.3 g. how many significant figures are justified in...
- C Chemistry Why is it not to use resolidified sample in melting point determination...
- H History In korea, the three kingdoms were at peace and worked well with one another. true or false...

Ответ:
pCH₄ = 105.1 - 0.42 = 104.68 torr
pI₂ = 7.96 -0.42 = 7.54 torr
pCH₃I = 0.42 torr
pHI = 0.42 torr
Explanation:
Kp is the equilibrium constant for the partial pressure of the gases in the reaction, and it is calculated for a general equation:
aA(g) + bB(g) ⇄ cC(g) + dD(g)
CH₄(g) + I₂(g) ⇄ CH₃I(g) + HI(g)
105.1 torr 7.96 torr 0 0 initial partial pressure
-x -x +x +x react
105.1-x 7.96-x x x equilibrium
Then:
x² = 0.1891 - 0.0255x -2.26x10⁻⁴x²
0.9997x² + 0.0255x - 0.1891 = 0
Using Bhaskara's rule:
Δ = (0.0255)² - 4x(0.9997)x(-0.1891)
Δ = 0.7568
Using only the positive term, x = 0.42 torr.
So,
pCH₄ = 105.1 - 0.42 = 104.68 torr
pI₂ = 7.96 -0.42 = 7.54 torr
pCH₃I = 0.42 torr
pHI = 0.42 torr
Ответ:
The given information gives us the clue that deuterium and tritium react together. We need to find the balanced nuclear reaction of deuterium and tritium; however, first, let me explain you what deuterium and tritium are.
Deuterium and tritium are two isotopes of hydrogen having atomic mass of 2 and 3 respectively. Deuterium is represented by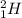 , whereas tritium is written as
, whereas tritium is written as 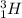 .
.
When deuterium and tritium collide together, they form Helium atom, which is written as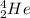 , and also release energy (and shoot out one neutron).
, and also release energy (and shoot out one neutron).
Hence, the balanced nuclear reaction will be the following:
-i