alonzob2367
04.11.2020 •
Chemistry
Solve the following problems on ideal gas equation. Practice makes perfect.
1. A toy balloon filled Analyze
with air has an
internal pressure of
1.25 atm and a
Calculate
volume of 2.50 L.
How many moles of
gas does the balloon
Answers: 1., 0.130 mol, 2.,0,012 mol, 3., 26 220 torr, 4., 1.07 atm
hold? (Assume T =
285 K)
Final Answer
2. A certain quantity of Analyze:
argon gas is under 16
torr pressure at 253K
in a 12 L vessel. How Calculate
many moles of argon
is present?
Final Answer
3. If 11.00 moles of HC1 Analyze:
gas occupies 15.00 L
at 300.0 °C, what is
its pressure in tort? Calculate
Final Answer
Analyze
4. What would be the
pressure of 6.40 g
oxygen gas in a
Solved
Show answers
More tips
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
- H Health and Medicine 10 Tips for Avoiding Vitamin Deficiency...
Answers on questions: Chemistry
- C Chemistry Why does an ionic bond transfer electrons instead of sharing?...
- C Chemistry In the following reaction, which element or compound is the oxidizing agent? I NEED HELP ASAP...
- C Chemistry The average kinetic energy of molecules is proportional to the temperature of the gas molecules (in kelvin). true false...
- C Chemistry Consider sodium oxide, na2o. choose the best explanation for the subscript, 2, from the list provided....
- C Chemistry What is the new boiling point if 25 g of nacl is dissolved in 1.0 kg of water...
- C Chemistry Which element will attract electrons more strongly—fluorine or carbon?...
- M Mathematics Diane has $814 in her checking account. If she writes five checks for a total of $287, how much does she have left in her account?...
- E English Which statement best describes the purpose of the first sentence in this paragraph...
- M Mathematics COMPUTE THE PROBABILITY. If, in a typical work day, a staff member receives 34 emails, what is the probabilitythat in a 5-day work week she will receive less than 175 emails?...
- M Mathematics Arrange the fractions from the largest to the smallest....

Ответ:
0.012mol
Explanation:
1.
Given parameters:
Pressure in the balloon = 1.25atm
Volume of the balloon = 2.5L
Unknown:
Number of moles = ?
Solution:
We are going to used the combined gas law to solve this problem.
PV = nRT
P is the pressure
V is the volume
n is the number of moles
R is the gas constant = 0.082atmdm³mol⁻¹K⁻¹
Insert the parameters and solve;
1.25 x 2.5 = n x 0.082 x 285
n = 0.13mol
The number of moles is 0.13mol
2.
Given parameters:
Pressure = 16torr;
to atm is ;
760torr = 1atm
16torr =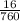 = 0.02atm
= 0.02atm
Volume = 12L
Temperature = 253K
Using the ideal gas law;
PV = nRT
0.02x12 = n x 0.082 x 253
n = 0.012mol
Ответ:
Bobby’s interquartile range is 113, while Amelia’s is 179. A lower interquartile range means Bobby’s scores are closer to the median.
Step-by-step explanation:
A lower interquartile range is clustered closer to the median than a higher interquartile range.