The reaction 3 A + 2 B + 4 C → products is known to be second order in A, first order in B and independent of the concentration of C. What is the value of the rate constant for this reaction if its rate is 0.771 M · s−1 when [A] = 5.00 M, [B] = 7.00 M, and [C] = 3.00M?Answer in units of M−2· s−1.
Solved
Show answers
More tips
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
- F Family and Home Why Having Pets at Home is Good for Your Health...
- H Health and Medicine How to perform artificial respiration?...
Answers on questions: Chemistry
- C Chemistry The gradual changes that have occurred in the earth’s structure during the past 4.5-5 billion years is termed . Please fill in the blank it’s due at 11:30 and I need...
- M Mathematics What s the circumference of a circle with a diameter of 12 ft? a. 264 ft. b. 84 ft. c. 19 ft. d. 37.7 ft....
- M Mathematics Frozen picture give me will give you brainest...
- S Spanish Choose the answer that correctly completes the sentence. él astronauta. somos es eres soy...

Ответ:
8)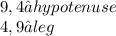
6)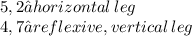
5)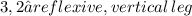
Step-by-step explanation:
Obviously, use the Pythagorean Theorem for all of them:
8)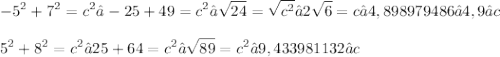
6)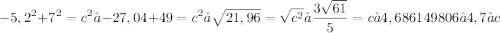
5)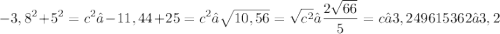
Split the isosceles base in half to get two identical legs of 3,8.
I am joyous to assist you anytime.