zuleromanos
16.07.2019 •
Chemistry
The vapor pressure of water is 23.76 mm hg at 25°c. how many grams of urea, ch4n2o, a nonvolatile, nonelectrolyte (mw = 60.10 g/mol), must be added to 238.2 grams of water to reduce the vapor pressure to 23.22 mm hg ? water = h2o = 18.02 g/mol.
Solved
Show answers
More tips
- W Work and Career Where can you learn to be a flight attendant?...
- G Goods and services How to Properly Calculate the Power of Your Air Conditioner?...
- F Food and Cooking Effective Methods to Organize Videos in your iPad According to Content...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
Answers on questions: Chemistry
- M Mathematics What is the y-intercept of the line whose equation is y = x - 3?...
- M Mathematics Determine the value of -14.1 times -7.33...
- M Mathematics HELP PLEASE AND THANKS I WILL MARK U AS BRAINLIEST!!...
- M Mathematics Pls help I will not let me add the screenshot so I m going to try and explain. so there is a rectangle the top measures 10m and the side measures 6m then there is a...

Ответ:
18.700 g
Explanation:
As the urea is a nonvolatile and nonelectrolyte solute, it will reduce the vapor pressure of the solution according to:
Where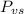 is the vapor pressure of the solution,
is the vapor pressure of the solution, 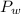 is the vapor pressure of the pure water, and
is the vapor pressure of the pure water, and  is the molar fraction of water. This equation applies just for that kind of solutes and at low pressures (23.76 mmHg is a low pressure).
is the molar fraction of water. This equation applies just for that kind of solutes and at low pressures (23.76 mmHg is a low pressure).
From the equation above lets calculate the water molar fraction:
So, the molar fraction of the urea should be:
Then, calculate the average molecular weight:
The molar fraction of urea is:
Solving for x,
Ответ: