Two aqueous solutions are prepared: 1.00 m Na2CO3 and 1.00 m LiCl. Which of the following statements is true?
A. The Na2CO3 solution has a higher osmotic pressure and higher vapor pressure than the LiCl solution.
B. The Na2CO3 solution has a higher osmotic pressure and higher boiling point than the LiCl solution.
C. The Na2CO3 solution has a lower osmotic pressure and lower vapor pressure than the LiCI solution.
D. The Na2CO3 solution has a lower osmotic pressure and higher boiling point than the LiCl solution.
E. None of these statements is true.
Solved
Show answers
More tips
- A Auto and Moto How many blood alcohol level units are allowed in Russian traffic laws?...
- G Goods and services Kogda zhdatt Iphone 5? The Latest News and Rumors...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Chemistry
- C Chemistry Determine the number of moles of iodine that reacts with 50g of aluminum...
- M Mathematics Write the English phrase as an algebraic expression. Then, simplify the expression. Let x represent the number. The difference between the product of five and a number and three...
- H Health Order the following steps to describe the process of how a body moves: the brain sends stimulation to the muscle the less stable bone moves towards the more stable bone around the...
- C Chemistry Which reaction will most likely take place?...
- M Mathematics Disney held a breakfast for parents and their children to eat with Mickey Mouse. Adult tickets cost $17.95 and children tickets cost $12.95. Disney made $7,355 in ticket sales from...

Ответ:
The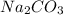 solution has a higher osmotic pressure and higher boiling point than LiCl solution.
solution has a higher osmotic pressure and higher boiling point than LiCl solution.
Explanation:
As concentrations of two aqueous solutions are same therefore we can write:
where ,
,  and
and  are lowering of vapor pressure, elevation in boiling point and osmotic pressure of solution respectively.
are lowering of vapor pressure, elevation in boiling point and osmotic pressure of solution respectively.  is van't hoff factor.
is van't hoff factor.
Here both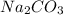 and LiCl are strong electrolytes.
and LiCl are strong electrolytes.
So, and
and 
Hence, lowering of vapor pressure, elevation in boiling point and osmotic pressure will be higher for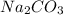 solution.
solution.
Therefore the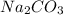 solution has a higher osmotic pressure and higher boiling point than LiCl solution.
solution has a higher osmotic pressure and higher boiling point than LiCl solution.
Ответ:
k thx :)
Explanation: