Which solvent will nacl, sodium chloride or table salt, will be more soluble in? nacl will be more soluble in kerosene because the non polar structure of kerosene allows the smaller lons to infiltrate the structure of the larger compound and therefore dissolve. nacl will be more soluble in kerosene because kerosene can dissolve all compounds. nacl will be equally soluble in both solvent. nal will be more soluble in water, because water is polar and can separate the cation and anion in the lonic compound, creating very strong ion dipole attractions
Solved
Show answers
More tips
- C Computers and Internet Е-head: How it Simplifies Life for Users?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Chemistry
- C Chemistry A particle that travels around the nucleus of an atom in orbitals is called a(n) quark. proton. neutron. electron....
- C Chemistry When force are ____ the object does not move...
- C Chemistry A force between an object and the surface it is moving over, is known as what? power O velocity O force O friction...
- C Chemistry Infer: Some powders produce little to no temperature change when added to water. Find the powder that has the smallest effect on the temperature of the resulting...
- M Mathematics Greg has a 20% chance of being selected as the president of the school sports club and a 90% chance of being elected editor of the school magazine. what is the probability...
- H History True or false all sumerian cities built ziggurats at the same time...
- M Mathematics If the velocity of a car 45km/h wes how far can it travel in 0.5 hours...
- E English Practicing good personal hygiene, such as keeping your nails clean and trimmed, will reduce the risk of:...
- S Spanish Select the answer that best completes the sentence. papel es blanco. la los las el...
- E English How does bierce develop the theme time can be subjective ?...

Ответ:
Option (d) is the correct answer.
Explanation:
It is known that NaCl is an ionic compound as it is formed by transfer of an electron from sodium atom to chlorine atom.
Since, an ionic bond is a bond formed due to transfer or donation of an electron from one atom to another.
Also, ionic compounds are soluble in polar solvents because like dissolves like.
So, when NaCl is added into water then it will dissociate into ions as and
and 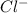 . As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.
. As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.
Whereas kerosene comes from petroleum (non-polar hydrocarbon) therefore, kerosene is a non-polar solvent. Hence, NaCl will not dissolve in it.
Thus, we can conclude that NaCl will be more soluble in water, because water is polar and can separate the cation and anion in the lonic compound, creating very strong ion dipole attractions.
Ответ:
because when something BURNS, it turns into NEW substances like smoke and smog.