You select a 150.0 ml volumetric flask to which you add 80.00 mL of a 2.30 M solution of the weak acid you just selected. To finish preparing your buffer you must now add the sodium salt of the conjugate base. How many grams of the sodium salt of your conjugate base must you add so that when you finally fill the flask to the mark with deionized water the buffer will have a pH of 8.50.
Solved
Show answers
More tips
- C Computers and Internet Dropbox: What is it and How to Use it...
- H Health and Medicine How to Increase Hemoglobin in the Blood...
- A Animals and plants How to Store Carrots: Tips for Homeowners...
- L Legal consultation Juvenile Justice: Who Needs It?...
- F Family and Home How to Choose the Best Diapers for Your Baby?...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
Answers on questions: Chemistry
- C Chemistry Why is there no sound in outer space? PLEASE HELP IF YOU WANT BRAINLEIST AND A LIKE ON URE COMMENT! A. The speed of sound is too slow for space.B. There is no medium...
- B Biology Following a forest fire in Yosemite National Park, trees start to re-grow throughout the area. What kind of succession occurred here? How do you know?...
- S Social Studies Summarize Georgia s contributions and how they benefited the state. Help plz...
- S Social Studies You have been asked by your principal to recommend one course which will help you prepare for the job you want in the future. Write an essay to explain to your principal...
- M Mathematics Of equations to answer the questions. 2x + 3y = 3 y = 8 - 3x The value of y from the second equation is substituted back into the first equation. What is the resulting...
- M Mathematics Si un albañil tarda en tres días levantar una barda si 10 albañiles al mismo ritmo ¿que tiempo se tardarán en levantar la misma barda? para hoy...
- M Mathematics Help please and thank you...
- M Mathematics Help me please I don t get it...
- B Biology Write a paragraph where you assume that all the peas in the pod had the same genes for size. In a brief discussion below, describe how the environment within the pea...
- P Physics A bullet at 800 m/s horizontally hits a target that is 180m away. How far does the bullet fall before it hits the target?...

Ответ:
7.33 grams of NaOH is the amount of conjugate base added.
Explanation:
given that:
total volume = 150 ml
Vacid = 80 ml
Macid = 2.30 M
Vbase= 150-80= 70ml
Mbase=?
atomic mass of NaOH = 39.99 grams/mole
formula for titration
Macid X Vacid = Mbase X Vbase
putting the values in the equation:
Mbase =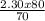
= 2.62M is the molarity of NaOH
Number of moles of
M =
2.62 x 0.07 = number of moles
0.1834 moles of NaOH is there, to know the mass formula used is
mass = number of moles x atomic mass
= x 0.1834
= 7.33 grams of NaOH is the amount of conjugate base added.
Ответ: