YSGabino2991
17.03.2020 •
Chemistry
You take an aspirin tablet (a compound consisting solely of carbon, hydrogen, and oxygen) with a mass of 1.00 g, burn it in air, and collect 2.20 g of carbon dioxide and 0.400 g water. The molar mass of aspirin is between 170 and 190 g/mol. The molecular formula of aspirin is
Solved
Show answers
More tips
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
- A Art and Culture When Will Eurovision 2011 Take Place?...
- S Style and Beauty How to Choose the Perfect Hair Straightener?...
Answers on questions: Chemistry
- C Chemistry When the equation Ca3(PO4)2+HNO3→Ca(NO3)2+H3PO4 is balanced, the smallest whole number coefficient for HNO3 is ...
- C Chemistry Pleasee help me with this :)...
- C Chemistry Which alkali is used to balance ph of human stomach...
- C Chemistry How many cg/m² is 0.0044 mg/mm²?...
- C Chemistry Is a solution in which (OH) = 1.0 10-1M acidic, basic, or neutral?...
- C Chemistry How does an atom-88 become a strontium ion with a +2 charge?...
- C Chemistry Melting ice is an example of which of the following? a- endothermic reaction b- chemical reaction c- isothermic reaction d- exothermic reaction...
- C Chemistry List the following bonds in order of increasing ionic character: csf, clcl, brcl, sic...
- C Chemistry Chemistry as level what does di substituted mean...
- S Social Studies What does the word history mean...

Ответ:
The formula of aspirin =
Explanation:
Mass of water obtained = 0.400
Molar mass of water = 18 g/mol
Moles of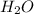 = 0.400 g /18 g/mol = 0.0222 moles
= 0.400 g /18 g/mol = 0.0222 moles
2 moles of hydrogen atoms are present in 1 mole of water. So,
Moles of H = 2 x 0.0222 = 0.0444 moles
Molar mass of H atom = 1.008 g/mol
Mass of H in molecule = 0.0444 x 1.008 = 0.0448 g
Mass of carbon dioxide obtained = 2.20 g
Molar mass of carbon dioxide = 44.01 g/mol
Moles of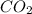 = 2.20 g /44.01 g/mol = 0.05 moles
= 2.20 g /44.01 g/mol = 0.05 moles
1 mole of carbon atoms are present in 1 mole of carbon dioxide. So,
Moles of C = 0.05 moles
Molar mass of C atom = 12.0107 g/mol
Mass of C in molecule = 0.05 x 12.0107 = 0.6005 g
Given that the aspirin acid only contains hydrogen, oxygen and carbon. So,
Mass of O in the sample = Total mass - Mass of C - Mass of H
Mass of the sample = 1.00 g
Mass of O in sample = 1.00 - 0.6005 - 0.0448 = 0.3547 g
Molar mass of O = 15.999 g/mol
Moles of O = 0.3547 / 15.999 = 0.0222 moles
Taking the simplest ratio for H, O and C as:
0.0444 : 0.0222 : 0.05
= 8 : 4 : 9
The empirical formula is =
Molecular formulas is the actual number of atoms of each element in the compound while empirical formulas is the simplest or reduced ratio of the elements in the compound.
Thus,
Molecular mass = n × Empirical mass
Where, n is any positive number from 1, 2, 3...
Mass from the Empirical formula = 9×12 + 8×1 + 16×4= 180 g/mol
The molar mass of aspirin is between 170 and 190 g/mol
So,
Molecular mass = n × Empirical mass
170 < n × 180 < 190
⇒ n = 1
The formula of aspirin =
Ответ:
Be a Good Listener. ...
Don't Be Afraid to Make an Audible. ...
Don't Be Flawless, Be Authentic. ...
Be Passionate, Not Ruthless. ...
Be on The Front Line.
etc,