dreannaevans2
03.12.2019 •
Physics
When light with a frequency f1 = 547.5 thz illuminates a metal surface, the most energetic photoelectrons have 1.260 x 10^-19 j of kinetic energy. when light with a frequency f2 = 738.8 thz is used instead, the most energetic photo-electrons have 2.480 x 10^-19 j of kinetic energy
using these experimental results, determine the approximate value of planck's constant.
Solved
Show answers
More tips
- A Auto and Moto How many blood alcohol level units are allowed in Russian traffic laws?...
- G Goods and services Kogda zhdatt Iphone 5? The Latest News and Rumors...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
- S Style and Beauty Intimate Haircut: The Reasons, Popularity, and Risks...
Answers on questions: Physics
- P Physics 011 10.0 points A sound wave has a frequency of 798 Hz in air and a wavelength of 0.48 m. What is the temperature of the air? As- sume the velocity of sound at 0°C is 329 m/s....
- P Physics A 5-cm-high peg is placed in front of a concave mirror with a radius of curvature of 20 cm. USE THE ABOVE INFORMATION TO ANSWER QUESTION 3 TO 6 The focal length of a convex...
- P Physics If a ball of mass M is dropped from a height h onto a spring with spring constant k (whose equilibrium positions is at height 0), compresses the spring an additional distance...
- P Physics The Earth orbits the sun with a speed of about 67000 miles per hour. If the Earth was to suddenly stop, it would...
- P Physics Hola, Kevin: al enviar este formulario, el propietario podrá ver su nombre y dirección de correo electrónico. * Obligatorio The unit of charge is the ohr (4 puntos) An object...
- P Physics Calculate the voltage difference in a circuit that has a resistance of 24 if the current is 0.50 a...
- P Physics What is the speed, in m/s, of a wave on a cord if it has a wavelength of 4m and a period of 0.5 s...
- P Physics The train speeds passed holly at 70 km/h north ward. this spider crawls past lukes foot in the same direction at 30 m/h. what is the velocity of the spider relative to holly?...
- H Health How are food-contact surfaces to be handled between uses A. Wash, rinse, and sanitize B. Wipe down with cleaner C. Clean and rinse D.Wipe down with cleaner and sanitize...
- B Biology Bauxite is a non-renewable resource since it * has a limited range of uses * cannot be readily replaced in nature * requires minimal processing after extraction * is found in...

Ответ:
The approximate value of Planck's constant is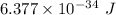
Explanation:
Given that,
Frequency
Kinetic energy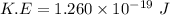
Frequency
Kinetic energy
We need to calculate the approximate value of Planck's constant
Using formula of change in energy
Hence, The approximate value of Planck's constant is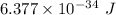
Ответ:
1. The resulting image is formed at 2F
2. The image formed is real and inverted
3. Magnification is 1
Explanation:
1. The image is formed at 2F
When an object is placed at point 2F of a convex lens, the resulting image will also be located at the point 2F on the other side of the lens.
This to say object length is equal to image length.
Using Equations;
u = object distance from the mirror = 2F
v = image distance from the mirror
f = focal length
substitute 2F for u
Therefore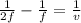
And v = -2f
i.e Image is formed at 2F
2. The image formed is real and inverted;
Real image means the image can be placed on a screen, i.e. light rays will converge at a location which exists in reality and this is accompanied by an inversion whereby an upright object forms an image that is upside-down.
3. Magnification is 1;
Magnification of a convex lens is the ratio of an object height to image height OR the ratio of distance of object to distance of image i.e.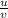
where u = 2f
and v = 2f
M = 1