How much heat is evolved in converting 1.00 mol of steam at 150.0 ∘C to ice at -45.0 ∘C? The heat capacity of steam is 2.01 J/(g⋅∘C) and of ice is 2.09 J/(g⋅∘C). Express your answer in units of kilojoules. Assume the system is at atmospheric pressure.
Solved
Show answers
More tips
- H Health and Medicine How does childbirth happen: everything future mothers need to know...
- H Horoscopes, Magic, Divination Is there a 13th Zodiac Sign?...
- H Health and Medicine Want to Lose Weight? Gain Muscle without Damaging Your Health!...
- F Family and Home Parquet or laminate, which is better?...
- L Leisure and Entertainment How to Properly Wind Fishing Line onto a Reel?...
- L Leisure and Entertainment How to Make a Paper Boat in Simple Steps...
- T Travel and tourism Maldives Adventures: What is the Best Season to Visit the Luxurious Beaches?...
- H Health and Medicine Kinesiology: What is it and How Does it Work?...
- O Other How to Choose the Best Answer to Your Question on The Grand Question ?...
- L Leisure and Entertainment History of International Women s Day: When Did the Celebration of March 8th Begin?...
Answers on questions: Chemistry
- C Chemistry When was magnesium discovered and how or who discovered it...
- C Chemistry Give the systematic name for this coordination compound [cr(h2o)4cl2]clo3...
- C Chemistry Hydrogen gas explosive within range of 4%-75% v/v. assuming that each student in your class produces 6 l of h2 and that the lab is not ventilated, is danger of an explosion?...
- C Chemistry Astandard solution of 0.243 m naoh was used to determine the concentration of a hydrochloric acid solution. if 46.33 ml of naoh is needed to neutralize 10.00 ml of the...
- C Chemistry If 57.0 ml of 1.0 m naoh solution is consumed to neutralize 25 ml of unknown hcl solution, find the concentration of the unknown hcl solution. hint: first you may have...
- C Chemistry Whats the temperature outside in florida...
- C Chemistry A thermos bottle contains 150 g of water at 4 oC. Into this bottle is placed 90 g of metal at 100 oC. After equilibrium is established, the temperature of the water and...
- C Chemistry Please help!!! When magnesium hydroxide, Mg(OH)2, is added to water it...Select one: a. falls to the bottom of the beaker undissolved. b. reacts with the water to form...
- C Chemistry What do you think life on earth will be like if chemistry had not been put to practical use...
- C Chemistry Ionic and covalent bonds help arrange atoms into unique structures for proteins like hemoglobin. Why is this necessary? (1 point) a. The ionic bonds alone in a protein...

Ответ:
Answer : The number of moles of oxygen formed are, 0.255 moles.
Explanation :
The given chemical reaction is:
This reaction is an unbalanced chemical reaction because in this reaction number of oxygen atoms are not balanced.
In order to balance the chemical equation, the coefficient '2' put before the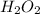 and
and  then we get the balanced chemical equation.
then we get the balanced chemical equation.
The balanced chemical reaction will be:
Now we have to calculate the number of moles of oxygen formed.
From the balanced chemical reaction we conclude that,
As, 2 moles of hydrogen peroxide react to give 1 mole of oxygen
So, 0.51 moles of hydrogen peroxide react to give mole of oxygen
mole of oxygen
Therefore, the number of moles of oxygen formed are, 0.255 moles.